Atom Models¶
There have been a number of atom models, I will try to document the important ones on this page.
Current Model (The Rutherford model)¶
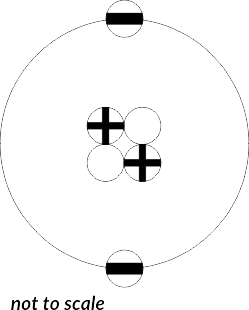
The most recent model, designed by Ernest Rutherford by using radioactivity, revolves around a nucleus, and shells containing electrons. The model consist of mostly empty space. The nucleus consists of protons and neutrons. The count of protons and neutrons is fixed for every atom. An atom has the same count of protons as electrons, however this changes in Ions, where the count of electrons differ from their atom.
Previous Models¶
Before Ernest Rutherford designed the model currently accepted, there were many other models.
Plum Pudding Model¶
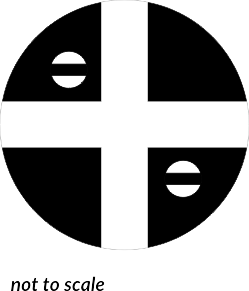
The Plum Pudding model describes the Atom as a positively-charged sphere, with negatively-charged electrons distributed throughout it.
Except as otherwise noted, the content of this page is licenced under the Creative Commons Attribution 4.0 License, and code samples are licensed under the Mozilla Public License 2.0.